Positive Material Identification (PMI) is one of the non-destructive testing (NDT) methods used to verify the material supplied is in compliance to the standards and specifications code. Most of our PMI customers are involved in Petrochemical, Oil & Gas Industries, Automotive and Power Generation. PMI is particularly used for high-quality metals like stainless steel and high alloy metals.
Positive Material Identification (PMI) is one of the non-destructive testing (NDT) methods used to verify the material supplied is in compliance to the standards and specifications code. Most of our PMI customers are involved in Petrochemical, Oil & Gas Industries, Automotive and Power Generation. PMI is particularly used for high-quality metals like stainless steel and high alloy metals.
How can PMI examination help Oil and Gas industries? The need for materials to be verified at the work-site is highly demanded by customers to ensure it meets standards and requirements. PMI is a portable method offering the best solution to determine or identify the alloy composition of the materials, even as easy as sorting the mix material and not required to sacrifice the sampling item. The American Petroleum Institute (API) requires that such testing is to be done in order to avoid material mix-ups, which can easily result in devastating accidents in the refinery environment.
Nusatek offers reliable PMI services both in laboratory and on-site with competent, experienced and well trained technical expertise. Our operating procedures and practices conform to international standards such as API-RP 578. PMI inspection is commonly used as it is an accurate and fast non-destructive technique for testing pipes, valves, bolts, flanges, rivets, welds, cladding or any other metal components to determine the alloy composition, both in-process and in stock.
We offer two methods for in-situ PMI as follows:
- PMI examination with XRF-machine (X-Ray Fluorescence)
- PMI examination with Arc Spark Emission Spectrography (OES)
Read More
List of PMI equipment we can provide


How does PMI examination help the Oil and Gas industry?
- The instrument is handheld and portable and ready to test materials anywhere, anytime, for example in-service, in-stock, in-process or inbound.
- Obtain accurate material alloy composition within a few minutes.
- Eliminate material mix-up during incoming and in-process.
- Re-verify material used in-process or fabrication compliance to code and standards.
XRF (X-Ray Fluorescence) Analyzer
- Sample preparation : No surface coating, clean from grease or contamination
- Size of sample : All range size of solid sample
- Chemical elements detect : All elements except Carbon content and light elements (P, S, Si)
This instrument uses an X-ray source to generate a beam of low energy radiation to excite the material under analysis. The electrons of the element in the alloy are exposed to the X-ray beam and are temporarily raised to an excited state. This can be accomplished with different X-ray excitation, either by a single isotope source or multiple-isotope sources (up to 3 sources) or by a tube X-ray.
An X-ray source generates a beam of primary X-rays which are focused into the alloy sample. Within the sample, the X-ray may be absorbed by atoms from various elements in the sample to cause atomic excitation of inner shell orbiting electrons. The excitation state of atoms are meta-stable, meaning they have a near instant transition from excitation to de-excitation by various processes including emission of fluorescent X-ray.
These fluorescent X-rays are characteristic in terms of their energies for each atomic transition occurring in the sample. These fluorescent emission of electromagnetic energy (X-ray) being unique for each atom, are like electromagnetic fingerprints that identify each individual element.
Therefore, the material under analysis emits a characteristic radiation spectrum which can be analyzed both qualitatively (X-ray spectrum) and quantitatively (% of element) to determine which elements are present and in what quantity.

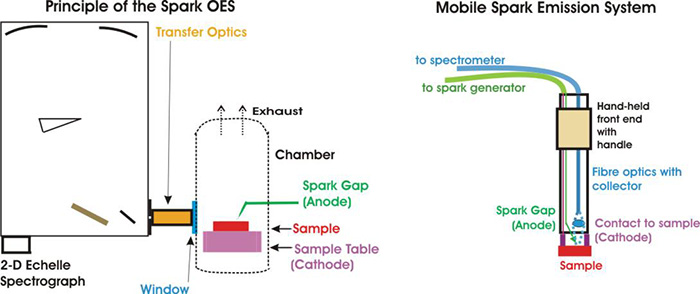
Arc Spark Emission Spectrography (OES) Analyzer
- Sample preparation : No surface coating, clean from grease or contamination and require surface finish grind
- Size of sample : flat surface on solid sample
- Chemical elements detect : All elements refer to CRM block reference

Figure 1: Burn mark after perform PMI test An Arc Spark Emission Spectrography (OES) Analyzer is an instrument to analyze metal alloys by using a technique where atoms in a sample are excited by energy that comes from a spark formed between the sample and an electrode. The energy of the spark causes the electrons in the sample to emit a characteristic spectrum of light from each element in the sample.
Basic theory of excitation or ionization of atoms
Matter is made of atoms, while atoms are made of nucleus and electrons. The nucleus is made of protons (+ve charge) and electron (-ve charge). When the sample is being sparked, it receives outside energy which causes excitation or ionization of atoms. The atom’s change in charge occurring through a loss or addition of electrons is called ionization. Because this state is higher, in terms of energy than atomic ground state, it is not stable. The ion attempts chemical reactions (molecule formation) within a few nanoseconds. The energy difference between the excited and the ground state, particularly for electron capture processes, is radiated in the form of light.
The characteristic spectrum of light from different elements are passed through a light guide to the optical analyzer, in the instrument. The light is dispersed into its spectral components and then measured and analyzed against a stored calibration curve. Through this analyzer, it can produce qualitative and quantitative analysis of the material composition with uncompromising accuracy.