 Chemical composition analysis is conducted for a wide range of purposes from material identification and characterization to quality control monitoring. The analysis provides valuable information about the elements of a test sample. Chemical composition analysis or elemental analysis can be qualitative (determining what elements are present), and it can be quantitative (determining how much of each are present). NUSATEK offers complete element analysis with quantitative and qualitative results.
Chemical composition analysis is conducted for a wide range of purposes from material identification and characterization to quality control monitoring. The analysis provides valuable information about the elements of a test sample. Chemical composition analysis or elemental analysis can be qualitative (determining what elements are present), and it can be quantitative (determining how much of each are present). NUSATEK offers complete element analysis with quantitative and qualitative results.
Chemical Composition Analysis Applications
- Verify conformance to a standard or specification (ASME, MIL, ASTM, etc)
- Evaluate of raw materials
- Detect impurities
- Identify the alloys
Chemical Composition Analysis is carried out using an Optical Emission Spectrometer that provides computerized processing and reporting results.
Applications
- Iron & steel and its alloys
- Aluminum and its alloys
- Copper and its alloys
- Nickel and its alloys
- Cobalt and its alloys
- Magnesium and its alloys
- Lead and its alloys
- Tin and its alloys
- Titanium and its alloys
- Zinc and its alloys
Read More
Chemical analysis is a significant test to verify that the material supplied meets the design or product requirement; or to identify the machinability, durability and weldability of the material. Chemical analysis is one of the important methods to support failure analysis investigation. Nowadays, the technologies in steel industries are more competitive and innovative. Manufacturers have come out with new varieties of specific modern steels to satisfy technological needs. These technologies may add, increase or reduce some critical elements in the making of the modern steel to develop lighter, stronger and more resistant to heat, chemicals, or the environment. The quality of the material shall be developed and controlled to ensure the composition meets the requirements.
Nusatek provides two (2) types of capabilities in order to verify or identify the chemical composition. We have the latest technologies for chemical composition analysis by using advanced Charge-Coupled Device (CCD) based Optical Emission Spectrometry (OES) and X-ray Fluorescence (XRF) methods. Each method has its own capabilities and advantages. We also provide a variety of Certified Reference Material (CRM) blocks to ensure all the chemical analysis results given are of true value and satisfactory.
Customers always demand for advanced technologies and accurate results. You can always consult us for the best method based on your needs. Our technical experts are well experienced in metal properties and are competent to perform the best method for chemical analysis testing.
Optical Emission Spectrometer
Atomic emission spectrometry (AES) is a method of chemical analysis that uses the intensity of light emitted from a flame, plasma, arc, or spark at a particular wavelength to determine the quantity of an element in a sample. The wavelength of the atomic spectral line gives the identity of the element while the intensity of the emitted light is proportional to the number of atoms of the element.
Spark or arc atomic emission spectroscopy is used for the analysis of metallic elements in solid samples. An electric arc or spark is passed through the sample, heating it to a high temperature to excite the atoms within it. The excited analyte atoms emit light at characteristic wavelengths that can be dispersed with a monochromator and detected. In the past, where the spark or arc conditions were typically not well controlled, the analysis for the elements in the sample was qualitative. However, modern spark sources with controlled discharges can be considered quantitative.
Principle of Optical Emission Spectrometry
Optical emission spectrometry involves applying electrical energy in the form of spark generated between an electrode and a metal sample, whereby the vaporized atoms are brought to a high energy state within a so-called “discharge plasma”.
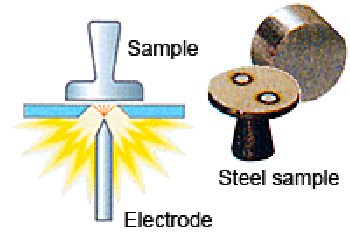
These excited atoms and ions in the discharge plasma create a unique emission spectrum specific to each element, as shown at right. Thus, a single element generates numerous characteristic emission spectral lines.
Therefore, the light generated by the discharge can be said to be a collection of the spectral lines generated by the elements in the sample. This light is split by a diffraction grating to extract the emission spectrum for the target elements. The intensity of each emission spectrum depends on the concentration of the element in the sample. Detectors (photomultiplier tubes) measure the presence or absence or presence of the spectrum extracted for each element and the intensity of the spectrum to perform qualitative and quantitative analysis of the elements.
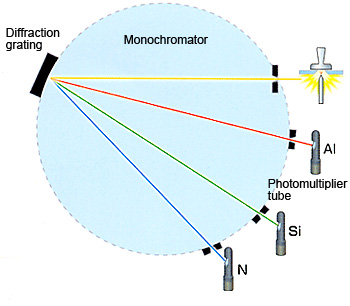
The Optical System
This spectrometer system is set-up with Paschen-Runge mounting. The operational technology uses the Solder Checker Application to detect the wavelength that produced upon ‘spark process. The new Charge-Coupled Device (CCD) based instrument with high resolution and Clear Spectrum technology detects the generated pulse and analyzes data which allows faster and more reliable results.
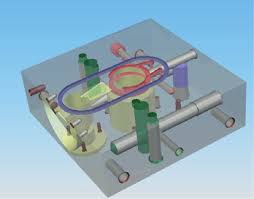
Figure 1: CCD system in OES equipment - Sample preparation : No surface coating, clean from grease orccontamination and require surface finish grind
- Size of sample : flat surface on solid sample (12mm – 100mm,width)
- Chemical elements detect : All elements refer to CRM block references

Figure 2: Example of burn mark revealed after chemical analysis test 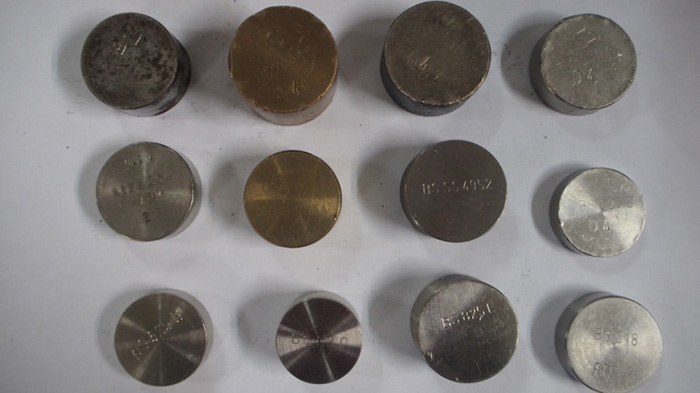
Figure 3: CRM block collection


