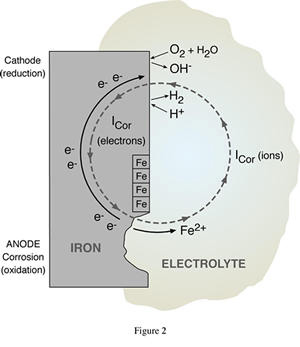
Corrosion is a natural process, which converts a refined metal to a more stable form, such as its oxide, hydroxide, or sulfide. It is the gradual destruction of materials (usually metals) by chemical reaction with their environment. Corrosion Tests are conducted to determine the resistance of a metal to chemical attack. Our laboratory is well organized, adequately equipped, and have competent staff who are trained to conduct these tests.
Corrosion is a natural process, which converts a refined metal to a more stable form, such as its oxide, hydroxide, or sulfide. It is the gradual destruction of materials (usually metals) by chemical reaction with their environment. Corrosion Tests are conducted to determine the resistance of a metal to chemical attack. Our laboratory is well organized, adequately equipped, and have competent staff who are trained to conduct these tests.
Our Corrosion Test services and capabilities are as follows:
- Pitting Corrosion Test as per ASTM G 48 Method A
- Ferric Chloride Corrosion Test as per ASTM A923 Practice C
- Intergranular Corrosion Test as per ASTM A262 Practice A, B and E
- Intergranular Corrosion Test as per ASTM G 28 Method A

Read More
Pitting Corrosion Test as per ASTM G 48 Method A
Pitting Corrosion is the localized corrosion of a metal surface confined to a point or small area that takes the form of cavities. Pitting corrosion is one of the most damaging forms of corrosion. Corrosion test is measured by weight loss per area of test sample.
These test methods determine the relative pitting resistance of stainless steel and nickel-base, chromium bearing alloys. Edges shall be rounded and a fine final polish is required at all surface exclude the weld cap and root area. The temperature and soaking time of test sample are based on customer’s requirement or upon request


Sample condition before soaking in Ferric chloride solution
Pitting was observed after soaking in Ferric chloride solution at 25°C until 24hours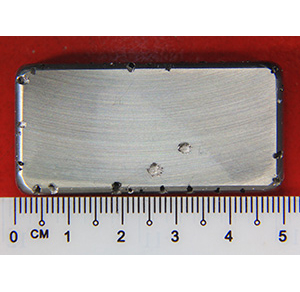

Test sample was soaked in Ferric chloride solution in incubator at 25°C
Ferric Chloride Corrosion Test as per ASTM A923 Practice C
This test method describes the procedure for conducting the ferric chloride corrosion test for detecting the presence of detrimental intermetallic phases in Duplex Stainless Steels.
Although this test method uses some similar equipment, this method should not be confused with Test Method G48. This test method does not determine the critical pitting temperature or test for the suitability for use in a particular environment.
It is defined for 2205 (S31803), that the test shall be at 25°C for 24 hours on a sample with uniform surface of at least equal to a 120-grit finish, or finer. Corrosion is measured by a weight loss that is converted to a corrosion rate. The corrosion test has the advantage of being relatively rapid, requiring 24 hours plus sample preparation time. It is not sensitive to size or orientation, provided that edge attack is not ignored.


Intergranular Corrosion Test as per ASTM A262 Practice A, B and E
Intergranular corrosion (IGC) is a selective attack in the vicinity of the grain boundaries of a stainless steel. It is a result of chromium depletion; mainly due to the precipitation of chromium carbides in the grain boundaries (this process is called sensitization).
ASTM A262 is Standard Practice For Detecting Susceptibility To Intergranular Attack In Austenitic Stainless Steels.
1. Practice A – Oxalic Acid Etch Test for Classification of Etch Structures of Austenitic Stainless Steel
Samples were prepared via metallography method ASTM E3 and etched with oxalic acid of electrolyte etching to reveal the structure. The structure was classified according to ASTM A262 Practice A (clause 11).

Test sample was etched using Oxalic Acid with electrolyte etching 
Ditch structure (sensitization) was observed in test sample after etched under high optical microscope 2. Practice B – Ferric Sulfate-Sulfuric Acid Test for Detecting Susceptibility to Intergranular Attack in Austenitic Stainless Steel
- This practice describes the procedure for conducting the test by boiling for 120 hours in ferric sulfate-50% sulfuric acid solution.
- A specimen representative of material to be evaluated is immersed in the boiling solution for a specified time. The resulting mass loss is converted to a corrosion rate, which is compared to a specified maximum value to attack expected of the grade of material being tested.


Specimen surface before soaking in boiling Ferric Sulfate-Sulfuric Acid solution 
Specimen surface before soaking in boiling Ferric Sulfate-Sulfuric Acid solution 3. Practice E – Copper-Copper Sulfate-Sulfuric Acid Test for Detecting Susceptibility to Intergranular Attack in Austenitic Stainless Steel
A suitable sample of an austenitic stainless steel is exposed to boiling acidified copper sulfate solution for 15 hours. After exposure in the boiling solution, the specimen is bent. Intergranular cracking or crazing is evidence of susceptibility.

Preparation 
Soaking Specimen 
Bend Specimen after soaking 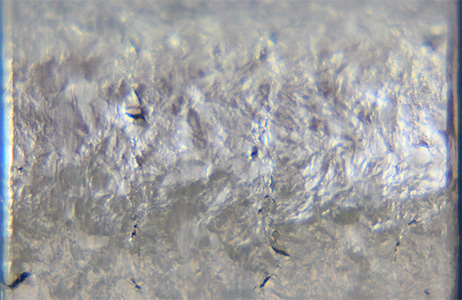
Examination of intergranular cracks and fissures on bend surface Intergranular Corrosion Test as per ASTM G 28 Method A
This test method describes the procedure for conducting the boiling ferric sulfate-50% sulfuric acid test which measures the susceptibility of certain nickel-rich, chromium-bearing alloys to intergranular corrosion.
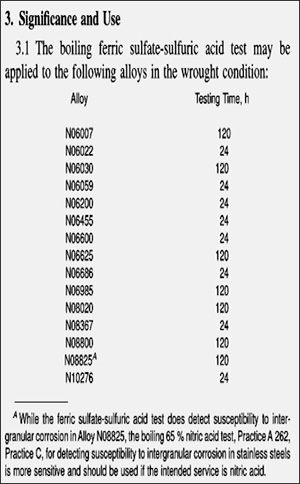
- This test method may be used to evaluate as-received material and to evaluate the effects of subsequent heat treatments. In the case of nickel-rich, chromium-bearing alloys, the test method may be applied to wrought and weldments of products. The test method is not applicable to cast products.
- The testing time for boiling ferric sulfate-sulfuric acid test depends on material of test sample to be evaluated as stated in ASTM G 28 Method A (Clause 3.1).
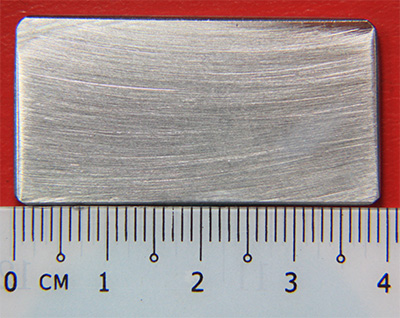
Test Specimen before soaking in Ferric Sulfate-50% Sulfuric Acid Solution 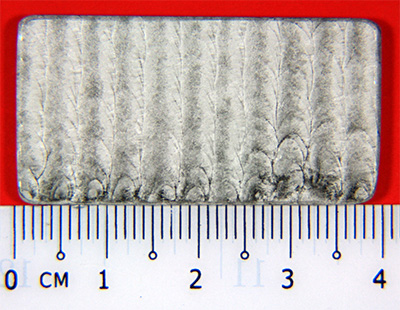
Test Specimen after soaking in Ferric Sulfate-50% Sulfuric Acid Solution